I was once asked a question during an interview that was worded like……….
The Many Hats of a Quality Manager
[fa icon="calendar'] Oct 28, 2013 6:00:40 AM / by Tricia Wood posted in Blog, Consistency, Quality, Quality Assurance, Quality Corner, Respect
Value of Raman Analysis in Pharmaceutical Investigations
[fa icon="calendar'] Oct 21, 2013 4:55:47 PM / by Admin posted in Blog, FTIR spectroscopy, Pharmaceutical, pharmaceutical drug products, Pharmaceutical QA/QC Investigations Webinars, Raman analysis, Raman spectral libraries, SEM/EDS, Recorded Webinars, microscopy
Original Event Date: Wednesday, November 20, 2013
Duration: 60 minutes
Presenters: Cara Plese, M.S., Scientist I, & Antonio Scatena, Laboratory Manager at Gateway Analytical
A Review of Some Tips for Condom Lubricant Analysis
[fa icon="calendar'] Oct 21, 2013 6:00:11 AM / by Cara Plese posted in Blog, condom lubricant analysis, evidence, Forensics, PDMS, sexual assault
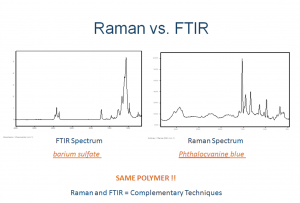
Comparison of Raman and FTIR Spectroscopy: Advantages and Limitations
[fa icon="calendar'] Oct 9, 2013 9:55:10 AM / by David Exline posted in Blog, Featured Articles, Fourier transform infrared spectroscopy, FTIR spectroscopy, Raman spectroscopy
Raman spectroscopy is an inelastic scattering phenomenon the probes molecular vibrations to provide a molecular fingerprint of materials. Currently, there are six major types of Raman spectroscopy in use today, which are: Spontaneous Raman Spectroscopy, Resonance Raman spectroscopy (RRS), Surface Enhanced Raman spectroscopy (SERS), Coherent Anti-Stokes Raman Scattering (CARS), Stimulated Raman Spectroscopy (SRS) and Spatially Offset Raman Spectroscopy (SORS).
Becoming Published for the First Time
[fa icon="calendar'] Oct 4, 2013 8:46:28 AM / by Rebekah Byrne posted in Blog, criminal trace evidence analysis, Inhalable drug products, Inhalation Magazine, Opportunity, pharmaceutical contamination, professional development
Opportunity is something that everyone looks for in a career. Opportunity does not just come in the form of ability to climb the promotional ladder; it comes in many forms. Whether that opportunity is providing employees with the ability to continue education and training or to encourage professional development through external presentations and publications, employers have the power to empower their team members with these types of opportunities. This empowerment generates morale and professional confidence in employees.
Q&A Recap from Spectral Analysis of Materials using Raman Chemical Imaging
[fa icon="calendar'] Sep 30, 2013 11:00:49 AM / by Admin posted in adhesives, Blog, coatings, failure analysis, glass, materials analysis, plastics, polymers, quality control, Spectral analysis
On September 18th, Rebekah Byrne, Scientist I and David Exline, Senior V.P. at Gateway Analytical hosted a webinar designed for professionals (e.g. Quality Control, Industrial Engineers) involved in the production and development of various materials, ranging from adhesives and polymers to plastics and glass. During this webinar, our presenters provided an overview of RCI and the type of data that can be generated using this technique. They discussed how this technique is currently being utilized in areas such as pharmaceuticals and provided details on how RCI can be beneficial for analyzing other types of materials, such as adhesives, polymers and coatings. They also discussed how analysis of materials using RCI can improve product development and failure analysis.
Continuous Quality Improvement: A Job for Everyone
[fa icon="calendar'] Sep 16, 2013 9:19:32 AM / by Admin posted in Blog, Quality Assurance, quality assurance, quality control, Quality Corner, quality management system, quality system
When everything within your quality system seems to be running like a well-oiled machine, it is easy to get stuck in a rut and perceive a lack of corrective actions or deviations as a signal that nothing needs to change. However, just because something works well does not negate the fact that it can be improved.
Q&A Recap for In Depth Analysis of Particle Contamination for Tablet and Semi-Solid Drug Products
[fa icon="calendar'] Sep 4, 2013 8:55:27 AM / by Admin posted in analytical testing, Blog, drug product, foreign particulate matter, manufacturing, particle contamination, Pharmaceutical, semi-solids, Tablets
On August 28th, Cara Plese, M.S., Scientist I and David Exline, Senior V.P. at Gateway Analytical hosted a webinar designed for professionals (e.g. Quality Control, Industrial Engineers) involved in the production and development of tablet and semi-solid drug products. During this webinar, our presenters provided examples of commonly found contaminants in tablet and semi-solid drug products and how these contaminants are introduced into the manufacturing process. They presented a basic multi-tiered analytical approach to characterize foreign particulate matter and how to solve particulate contamination issues using a scientific approach. The importance of taking a pro-active approach to quickly source and manage contaminant issues in the manufacturing process to reduce the risk of future contamination was also discussed.
Improve Employee Morale and Performance through Continued Training Opportunities
[fa icon="calendar'] Aug 30, 2013 12:54:38 PM / by Tricia Wood posted in Blog, employee loyalty, mentoring, morale, Quality Assurance, Quality Corner, training
In Depth Analysis of Particle Contamination for Tablet and Semi-Solid Drug Products
[fa icon="calendar'] Aug 28, 2013 12:00:17 AM / by Admin posted in analytical testing, Blog, drug product, foreign particulate matter, manufacturing, particle contamination, Pharmaceutical, Pharmaceutical QA/QC Investigations Webinars, Semi-Solid, tablet
Original Event Date: Wednesday, August 28, 2013
Duration: 60 minutes
Presenters: David Exline, Senior Vice President & Cara Plese, M.S., Scientist I at Gateway Analytical
Host: Tifanie Tiberio, Event Coordinator at Gateway Analytical