Presented at: 2014 AAPS Conference
Authors: Angela Flowers, Rebekah Byrne, Emily Landsperger, David Exline
Release Date: November 2014
Foreign Particulate and Glass Delamination Investigations using Automated Raman/LIBS
[fa icon="calendar'] Nov 19, 2014 1:29:23 AM / by Rebekah Byrne posted in 2014 AAPS Poster, analytical testing, automated Raman, Blog, foreign particulate, foreign particulate matter, glass delamination, particulate characterization, Posters & Presentations, Raman/LIBS
Q&A Recap for In Depth Analysis of Particle Contamination for Tablet and Semi-Solid Drug Products
[fa icon="calendar'] Sep 4, 2013 8:55:27 AM / by Admin posted in analytical testing, Blog, drug product, foreign particulate matter, manufacturing, particle contamination, Pharmaceutical, semi-solids, Tablets
On August 28th, Cara Plese, M.S., Scientist I and David Exline, Senior V.P. at Gateway Analytical hosted a webinar designed for professionals (e.g. Quality Control, Industrial Engineers) involved in the production and development of tablet and semi-solid drug products. During this webinar, our presenters provided examples of commonly found contaminants in tablet and semi-solid drug products and how these contaminants are introduced into the manufacturing process. They presented a basic multi-tiered analytical approach to characterize foreign particulate matter and how to solve particulate contamination issues using a scientific approach. The importance of taking a pro-active approach to quickly source and manage contaminant issues in the manufacturing process to reduce the risk of future contamination was also discussed.
In Depth Analysis of Particle Contamination for Tablet and Semi-Solid Drug Products
[fa icon="calendar'] Aug 28, 2013 12:00:17 AM / by Admin posted in analytical testing, Blog, drug product, foreign particulate matter, manufacturing, particle contamination, Pharmaceutical, Pharmaceutical QA/QC Investigations Webinars, Semi-Solid, tablet
Original Event Date: Wednesday, August 28, 2013
Duration: 60 minutes
Presenters: David Exline, Senior Vice President & Cara Plese, M.S., Scientist I at Gateway Analytical
Host: Tifanie Tiberio, Event Coordinator at Gateway Analytical
In Depth Analysis of Particle Contamination for Inhalable Drug Products
[fa icon="calendar'] Jun 4, 2013 6:05:49 PM / by Admin posted in analytical approach, Blog, dave exline, foreign particulate matter, Inhalable drug products, Medical Device Webinar Recordings, particle contamination, Pharmaceutical, Pharmaceutical QA/QC Investigations Webinars, Recorded Webinars
Original Event Date: Tuesday, June 4, 2013
Duration: 45 minutes
Presenters: David Exline, Senior Vice President at Gateway Analytical
Host: Shawn Wilhelm, Marketing Coordinator at Gateway Analytical
In Depth Analysis of Particle Contamination for Inhalable Drug Products
[fa icon="calendar'] Apr 2, 2013 2:15:49 PM / by Admin posted in analytical approach, Blog, dave exline, foreign particulate matter, Inhalable drug products, particle contamination, Pharmaceutical, Pharmaceutical QA/QC Investigations Webinars, Recorded Webinars
Original Event Date: Tuesday, June 4, 2013
Duration: 45 minutes
Presenters: David Exline, Senior Vice President at Gateway Analytical
Host: Shawn Wilhelm, Marketing Coordinator at Gateway Analytical
IFPAC Presentation: Forensic Analysis of Foreign Particles in Pharmaceutical Materials
[fa icon="calendar'] Feb 26, 2013 12:20:56 PM / by Rebekah Byrne posted in Blog, contaminants, foreign particulate matter, IFPAC, Pharmaceutical, pharmaceutical forensics
At this years IFPAC - 2013 27th International Forum and Exhibition Process Analytical Technology, I gave a presentation titled Forensic Analysis of Foreign Particles in Pharmaceutical Materials.
Forensic Analysis of Foreign Particles in Pharmaceutical Materials at the IFPAC Annual Meeting
[fa icon="calendar'] Jan 23, 2013 10:30:09 AM / by Rebekah Byrne posted in Blog, contamination investigations, deviation investigations, foreign particulate matter, non-conformance, Particle Identification in Pharmaceuticals, Pharmaceutical, pharmaceutical forensics
Conference: IFPAC - 2013 27th International Forum and Exhibition Process Analytical Technology
Location: Baltimore, Maryland, U.S.A.
Date: Wednesday, January 23rd during the PM VIII Session
Time: 1 – 5 pm
Webinar Recap and Q&A for Determining the Source of Contaminants in a Manufacturing Process
[fa icon="calendar'] Nov 29, 2012 12:50:24 PM / by Admin posted in Blog, foreign particulate matter, Pharmaceutical, pharmaceutical forensics, quality control, source determination
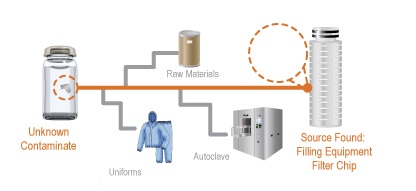
On October 30th, David Exline, Senior V.P. and Antonio Scatena, Laboratory Manager at Gateway Analytical hosted a webinar designed for laboratory and manufacturing personnel that encounter contaminants and foreign particulate matter in the manufacturing, process/product development and customer returns. This webinar discussed the process by which contaminants and foreign particles are isolated, identified, tracked to find the source of the issue, and then addressed to ensure that the issue does not arise again.