Chemically Specific Particle Sizing & Agglomerate Analysis Services
[fa icon="calendar'] May 15, 2014 1:56:56 PM / by Admin posted in aqueous suspension formulations, bioequivalence, Blog, chemically specific analysis, chemically specific particle sizing, Ingredient-Specific Particle Sizing, particle size distribution, particle sizing, pharmaceutical formulation, Raman chemical imaging, Videos
Gateway Analytical to Present with the FDA on Chemically Specific Analysis of Orally Inhaled Drug Products at the Respiratory Drug Delivery Conference in Puerto Rico
[fa icon="calendar'] Apr 24, 2014 6:00:13 AM / by Admin posted in Blog, chemically specific analysis, Drug Particle Agglomeration, FDA submission, Ingredient-Specific Particle Sizing, News Releases, OINDPs, Raman chemical imaging, RDD 2014
PITTSBURGH, April 24th, 2014 — Gateway Analytical announced today that they will be exhibiting and presenting at the upcoming Respiratory Drug Delivery (RDD) conference in Fajardo, Puerto Rico, May 4-8. RDD is an international conference that features in-depth presentations and discussions focused on the latest development in respiratory drug delivery science, in addition to industry networking. During the conference Gateway will be promoting their unique Raman chemical imaging services that provide drug developers with truly chemically specific particle characterization. This chemical specificity is the key to understanding the extend of agglomerations in orally inhaled drug products (OINDPs), which is critical for safety, stability and FDA submission.
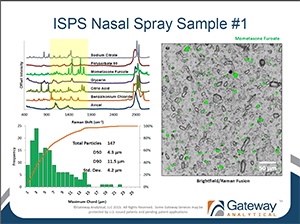
RDD Europe 2013 Workshop: Automated Ingredient Specific Particle Sizing
[fa icon="calendar'] Jun 12, 2013 9:00:42 AM / by Admin posted in bioequivalence, Blog, cGMP, Ingredient-Specific Particle Sizing, ISPS, particle characterization, particle sizing, Pharmaceutical
Gateway Analytical Senior Scientist Oksana Olkhovyk, Ph.D. recently presented at RDD Europe 2013 in Berlin, Germany. The workshop titled: "Automated Ingredient Specific Particle Sizing" is now available for download.
Working to Address the Critical Path Opportunity for Generic Nasal Spray Suspensions Q&A Recap
[fa icon="calendar'] Feb 15, 2013 11:34:20 AM / by Admin posted in Blog, Critical Path Opportunity, Generic nasal spray suspsension, Ingredient-Specific Particle Sizing, particle sizing, Pharmaceutical
On February 12th, Tracey Safran, Senior Account Manager and Oksana Olkhovyk, Senior Scientist at Gateway Analytical hosted a webinar for generic drug manufacturers of nasal spray suspensions discussing how the method of Ingredient-Specific Particle Sizing (ISPS) using Raman Chemical Imaging (RCI) developed by Gateway Analytical, can address the critical path opportunity (CPO) by providing the accurate and precise drug particle size measurement to demonstrate bioequivalence.
New Webinar Series for Scientist on Chemical Imaging June - Sept.
[fa icon="calendar'] May 24, 2012 1:50:33 PM / by Admin posted in aqueous suspension formulations, Blog, coating thickness, Controlled Release, controlled release, Ingredient-Specific Particle Sizing, inhalation drugs, method development, Pharmaceutical, pharmaceutical, pharmaceutical formulation, pharmaceutical products, polymers, Raman chemical imaging, semi-solids
Gateway Analytical will be offering a new webinar series for scientists, focused on chemical imaging, June though September of this year. This webinar series is designed for scientists developing and manufacturing various types of pharmaceutical products; including nasal, inhalation, semi-solid, solid, and controlled release drug delivery.
Preview: Joint Poster at Respiratory Drug Delivery 2012
[fa icon="calendar'] May 4, 2012 2:11:41 PM / by Admin posted in analytical testing, Blog, dry powder inhaler, Ingredient-Specific Particle Sizing, method validation, Nasal Drug Products, Nasal suspension PSD, Pharmaceutical, pharmaceutical, pharmaceutical formulation, Raman chemical imaging
This year at Respiratory Drug Delivery 2012, Dr. Oksana Olkhovyk will be presenting a poster with Christopher Vernall from the University of Bath which is located in the UK. This year’s poster topic, titled: “Investigation of the Microstructure of Combination Dry Powder Inhaler Formulations by Atomic Force Microscopy and Raman Chemical Imaging”, aims to investigate the microstructure of commercial Seretide® Accuhaler® using RCI and the cohesive-adhesive balance (CAB) approach to colloid probe AFM.