Chemically Specific Particle Sizing & Agglomerate Analysis Services
[fa icon="calendar'] May 15, 2014 1:56:56 PM / by Admin posted in aqueous suspension formulations, bioequivalence, Blog, chemically specific analysis, chemically specific particle sizing, Ingredient-Specific Particle Sizing, particle size distribution, particle sizing, pharmaceutical formulation, Raman chemical imaging, Videos
Gateway Analytical Acquires RapID System to Meet Growing Market Demand
[fa icon="calendar'] Jan 30, 2014 4:30:19 AM / by Admin posted in Blog, foreign particulate, News Releases, particle counting, particle identification, particle sizing, RapID
PITTSBURGH, January, 21 2014 — Gateway Analytical announced today that they have acquired the RapID SPE-ls raman.ID + metal.ID® system, the latest technology in automated particle identification. This acquisition enhances existing analytical service offerings to the pharmaceutical and materials science industries, and makes Gateway one of only two analytical service laboratories in the United States that can offer this advanced technology.
RDD Europe 2013 Workshop: Automated Ingredient Specific Particle Sizing
[fa icon="calendar'] Jun 12, 2013 9:00:42 AM / by Admin posted in bioequivalence, Blog, cGMP, Ingredient-Specific Particle Sizing, ISPS, particle characterization, particle sizing, Pharmaceutical
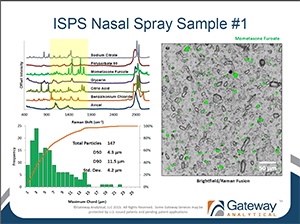
Gateway Analytical Senior Scientist Oksana Olkhovyk, Ph.D. recently presented at RDD Europe 2013 in Berlin, Germany. The workshop titled: "Automated Ingredient Specific Particle Sizing" is now available for download.
Working to Address the Critical Path Opportunity for Generic Nasal Spray Suspensions Q&A Recap
[fa icon="calendar'] Feb 15, 2013 11:34:20 AM / by Admin posted in Blog, Critical Path Opportunity, Generic nasal spray suspsension, Ingredient-Specific Particle Sizing, particle sizing, Pharmaceutical
On February 12th, Tracey Safran, Senior Account Manager and Oksana Olkhovyk, Senior Scientist at Gateway Analytical hosted a webinar for generic drug manufacturers of nasal spray suspensions discussing how the method of Ingredient-Specific Particle Sizing (ISPS) using Raman Chemical Imaging (RCI) developed by Gateway Analytical, can address the critical path opportunity (CPO) by providing the accurate and precise drug particle size measurement to demonstrate bioequivalence.