Chemically Specific Particle Sizing & Agglomerate Analysis Services
[fa icon="calendar'] May 15, 2014 1:56:56 PM / by Admin posted in aqueous suspension formulations, bioequivalence, Blog, chemically specific analysis, chemically specific particle sizing, Ingredient-Specific Particle Sizing, particle size distribution, particle sizing, pharmaceutical formulation, Raman chemical imaging, Videos
A 2013 AAPS Annual Meeting Recap
[fa icon="calendar'] Nov 25, 2013 9:45:56 AM / by Admin posted in AAPS, bioequivalence, Blog, chemically specific, drug manufacturing, Pharmaceutical, pharmaceutical formulation, sem-eds
For the third consecutive year Gateway had the pleasure of exhibiting at the AAPS Annual Meeting, which was held this year in San Antonio, at the very large Henry B. Gonzalez Convention Center. AAPS members and attendees come from a wide range of areas within pharmaceutical drug development and biopharmaceuticals. In addition to offering free popcorn daily and an open bar on Tuesday night, this year, our focus was to promote analytical services that not only support drug product formulation development but also address manufacturing issues such as particulate contamination and source determination.
RDD Europe 2013 Workshop: Automated Ingredient Specific Particle Sizing
[fa icon="calendar'] Jun 12, 2013 9:00:42 AM / by Admin posted in bioequivalence, Blog, cGMP, Ingredient-Specific Particle Sizing, ISPS, particle characterization, particle sizing, Pharmaceutical
Gateway Analytical Senior Scientist Oksana Olkhovyk, Ph.D. recently presented at RDD Europe 2013 in Berlin, Germany. The workshop titled: "Automated Ingredient Specific Particle Sizing" is now available for download.
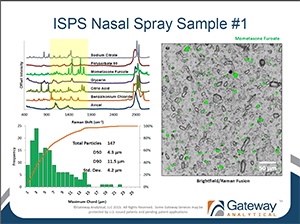
Working to Address the FDA Critical Path Opportunity for Generic Nasal Spray Suspensions
[fa icon="calendar'] Jan 22, 2013 3:27:15 PM / by Admin posted in bioequivalence, Blog, cGMP laboratory, Critical Path Opportunity, Generic nasal spray suspsension, Ingredient-Specific Particle Sizsing, Pharmaceutical, Pharmaceutical Webinars, Raman chemical imaging, Recorded Webinars
Helping Generic Manufacturers Prove Bioequivalence of Suspension Nasal Sprays
[fa icon="calendar'] Apr 20, 2012 10:30:45 AM / by David Exline posted in analytical testing, aqueous suspension formulations, bioequivalence, Blog, critical path opportunities, FDA, in vitro, in vivo, Nasal Drug Products, Nasal suspension PSD, Pharmaceutical, pharmaceutical, pharmaceutical formulation, suspension nasal sprays
In 2004, the FDA launched the Critical Path Initiative as a national strategy to drive innovation in development, evaluation and manufacturing of medical products1. As part of this initiative, the FDA identified a number of Critical Path Opportunities (CPOs) for generic drugs. Namely the CPO for Bioequivalence of Nasal Sprays, which identifies a need for direct measurement of particle size equivalence in suspension nasal sprays2.