At this years IFPAC - 2013 27th International Forum and Exhibition Process Analytical Technology, I gave a presentation titled Forensic Analysis of Foreign Particles in Pharmaceutical Materials.
IFPAC Presentation: Forensic Analysis of Foreign Particles in Pharmaceutical Materials
[fa icon="calendar'] Feb 26, 2013 12:20:56 PM / by Rebekah Byrne posted in Blog, contaminants, foreign particulate matter, IFPAC, Pharmaceutical, pharmaceutical forensics
David Exline, President
[fa icon="calendar'] Jan 29, 2013 9:50:34 AM / by David Exline posted in analytical instrumentation, analytical testing, Authors, Blog, chemical imaging, crime scene investigation, fluorescence microscopy, forensic expert, FTIR, GMP, hyperspectral imaging, light microscopy, pharmaceutical, pharmaceutical forensics, Raman chemical imaging, Raman spectroscopy, spectroscopy, trace evidence, USP testing methods
Forensic Analysis of Foreign Particles in Pharmaceutical Materials at the IFPAC Annual Meeting
[fa icon="calendar'] Jan 23, 2013 10:30:09 AM / by Rebekah Byrne posted in Blog, contamination investigations, deviation investigations, foreign particulate matter, non-conformance, Particle Identification in Pharmaceuticals, Pharmaceutical, pharmaceutical forensics
Conference: IFPAC - 2013 27th International Forum and Exhibition Process Analytical Technology
Location: Baltimore, Maryland, U.S.A.
Date: Wednesday, January 23rd during the PM VIII Session
Time: 1 – 5 pm
Gateway Analytical Interview on Pittsburgh Technology Council’s TechVibe Radio
[fa icon="calendar'] Jan 8, 2013 11:04:11 AM / by Admin posted in analytical testing, Blog, forensic expert, forensics, Pharmaceutical, pharmaceutical, pharmaceutical forensics
Recently on December 22nd, Dave Exline, Senior V.P. at Gateway Analytical stopped by the Pittsburgh Technology Council’s TechVibe Radio to talk on-air with Audrey Russo and Jonathan Kersting about Gateway Analytical and its services.
Webinar Recap and Q&A for Laboratory Methods for Failure Analysis of Pharmaceutical Products
[fa icon="calendar'] Dec 5, 2012 12:05:39 PM / by Admin posted in Blog, failure analysis, nonconformance, Pharmaceutical, pharmaceutical forensics, problem solving, quality assurance, quality control, quality management system
On November 14th, Dave Exline, Senior V.P. and Rebekah Wagurak, Forensic Scientist at Gateway Analytical hosted a webinar designed for laboratory and manufacturing personnel that are responsible for quality assurance and quality control of processes in the manufacturing of pharmaceuticals. This webinar discussed case studies and walked participants through the process of a failure analysis investigation, common methods used, and interpretation of analytical data to improve overall process development and quality control.
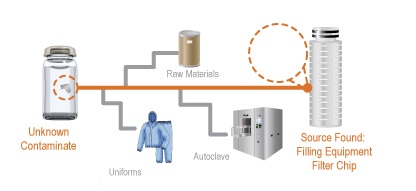
Webinar Recap and Q&A for Determining the Source of Contaminants in a Manufacturing Process
[fa icon="calendar'] Nov 29, 2012 12:50:24 PM / by Admin posted in Blog, foreign particulate matter, Pharmaceutical, pharmaceutical forensics, quality control, source determination
On October 30th, David Exline, Senior V.P. and Antonio Scatena, Laboratory Manager at Gateway Analytical hosted a webinar designed for laboratory and manufacturing personnel that encounter contaminants and foreign particulate matter in the manufacturing, process/product development and customer returns. This webinar discussed the process by which contaminants and foreign particles are isolated, identified, tracked to find the source of the issue, and then addressed to ensure that the issue does not arise again.
Gateway Analytical Receives Controlled Substance Registration Certificate from the Drug Enforcement Administration
[fa icon="calendar'] Jul 26, 2012 4:20:53 PM / by Rebekah Byrne posted in Blog, Controlled Substance Registration, DEA, drug scheduling, Pharmaceutical, pharmaceutical forensics
Gateway Analytical is proud to announce that we have obtained our Controlled Substance Registration Certificate issued by the United States Department of Justice Drug Enforcement Administration’s Office of Diversion Control.
Webinar Recap: Laboratory Methods for the Examination of Foreign Particulate Matter
[fa icon="calendar'] Apr 30, 2012 10:30:22 AM / by Admin posted in Blog, FTIR, FTIR spectroscopy, Pharmaceutical, pharmaceutical, pharmaceutical forensics, quality assurance, quality control, quality management system, Raman chemical imaging, SEM/EDS, source determination
On April 25th, 2012 we hosted a live webinar titled “Laboratory Methods for the Examination of Foreign Particulate Matter.” During the webinar our presenters, David Exline, Senior V.P. and Antonio Scatena, Scientist at Gateway Analytical, discussed current methods of particulate isolation, laboratory testing methods for identifying foreign particulates and the company's forensic approach to source determination. During the webinar we received a number of questions, below is a sample of the Q&A session at the end of the webinar.