On February 12th, Tracey Safran, Senior Account Manager and Oksana Olkhovyk, Senior Scientist at Gateway Analytical hosted a webinar for generic drug manufacturers of nasal spray suspensions discussing how the method of Ingredient-Specific Particle Sizing (ISPS) using Raman Chemical Imaging (RCI) developed by Gateway Analytical, can address the critical path opportunity (CPO) by providing the accurate and precise drug particle size measurement to demonstrate bioequivalence.
Working to Address the Critical Path Opportunity for Generic Nasal Spray Suspensions Q&A Recap
[fa icon="calendar'] Feb 15, 2013 11:34:20 AM / by Admin posted in Blog, Critical Path Opportunity, Generic nasal spray suspsension, Ingredient-Specific Particle Sizing, particle sizing, Pharmaceutical
Forensic Analysis of Foreign Particles in Pharmaceutical Materials at the IFPAC Annual Meeting
[fa icon="calendar'] Jan 23, 2013 10:30:09 AM / by Rebekah Byrne posted in Blog, contamination investigations, deviation investigations, foreign particulate matter, non-conformance, Particle Identification in Pharmaceuticals, Pharmaceutical, pharmaceutical forensics
Conference: IFPAC - 2013 27th International Forum and Exhibition Process Analytical Technology
Location: Baltimore, Maryland, U.S.A.
Date: Wednesday, January 23rd during the PM VIII Session
Time: 1 – 5 pm
Working to Address the FDA Critical Path Opportunity for Generic Nasal Spray Suspensions
[fa icon="calendar'] Jan 22, 2013 3:27:15 PM / by Admin posted in bioequivalence, Blog, cGMP laboratory, Critical Path Opportunity, Generic nasal spray suspsension, Ingredient-Specific Particle Sizsing, Pharmaceutical, Pharmaceutical Webinars, Raman chemical imaging, Recorded Webinars
Gateway Analytical Interview on Pittsburgh Technology Council’s TechVibe Radio
[fa icon="calendar'] Jan 8, 2013 11:04:11 AM / by Admin posted in analytical testing, Blog, forensic expert, forensics, Pharmaceutical, pharmaceutical, pharmaceutical forensics
Recently on December 22nd, Dave Exline, Senior V.P. at Gateway Analytical stopped by the Pittsburgh Technology Council’s TechVibe Radio to talk on-air with Audrey Russo and Jonathan Kersting about Gateway Analytical and its services.
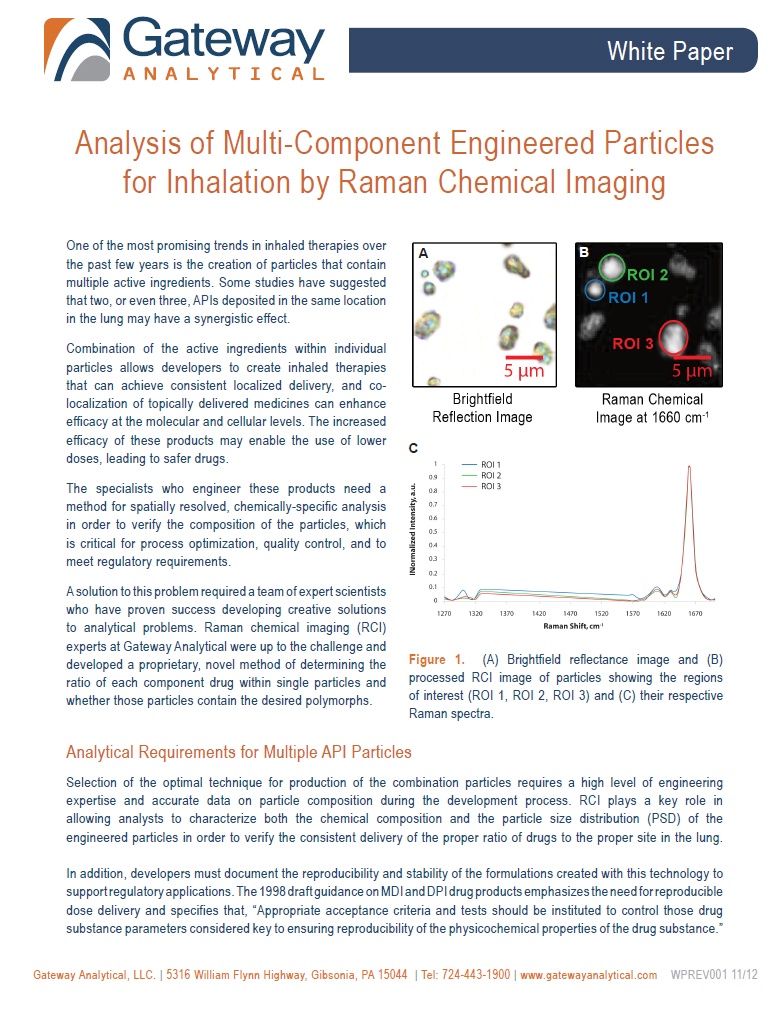
Using Raman Chemical Imaging to Analyze Inhaled Combination Therapies
[fa icon="calendar'] Dec 13, 2012 2:00:54 PM / by Oksana Olkhovyk posted in Articles & Publications, Blog, Pharmaceutical, White Papers
White Paper Preview:
Analysis of Multi-Component Engineered Particles for Inhalation by Raman Chemical Imaging
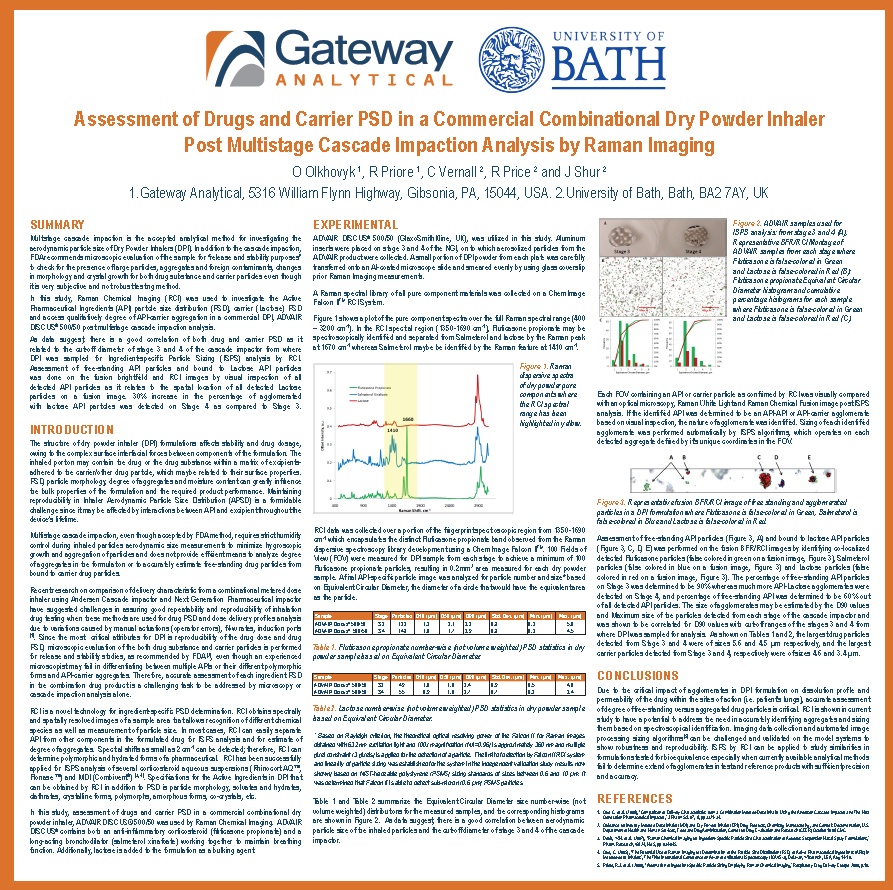
[fa icon="calendar'] Dec 11, 2012 10:30:57 AM / by Oksana Olkhovyk posted in Articles & Publications, Blog, Pharmaceutical, White Papers
White Paper Preview:
Assessment of Drugs and Carrier PSD in a Commercial Combinational Dry Powder Inhaler Post Multistage Cascade Impaction Analysis by Raman Imaging
[fa icon="calendar'] Dec 7, 2012 12:17:16 PM / by Oksana Olkhovyk posted in Blog, Pharmaceutical, Posters & Presentations
Presented at: Drug Delivery to the Lung 23
Authors: O Olkhovyk 1, R Priore 1, C Vernall 2, R Price 2 and J Shur 2
1.Gateway Analytical, 2.University of Bath
Release Date: Wed., December 5, 2012
Webinar Recap and Q&A for Laboratory Methods for Failure Analysis of Pharmaceutical Products
[fa icon="calendar'] Dec 5, 2012 12:05:39 PM / by Admin posted in Blog, failure analysis, nonconformance, Pharmaceutical, pharmaceutical forensics, problem solving, quality assurance, quality control, quality management system
On November 14th, Dave Exline, Senior V.P. and Rebekah Wagurak, Forensic Scientist at Gateway Analytical hosted a webinar designed for laboratory and manufacturing personnel that are responsible for quality assurance and quality control of processes in the manufacturing of pharmaceuticals. This webinar discussed case studies and walked participants through the process of a failure analysis investigation, common methods used, and interpretation of analytical data to improve overall process development and quality control.
Drug Delivery to the Lung 23 Poster Preview
[fa icon="calendar'] Nov 29, 2012 12:50:58 PM / by Oksana Olkhovyk posted in Blog, Pharmaceutical
Poster Title: Assessment of Drugs and Carrier PSD in a Commercial Combinational Dry Powder Inhaler Post Multistage Cascade Impaction Analysis by Raman Imaging
Date: Wed., December 5, 2012
Time: 3:30 pm - 5:00 pm
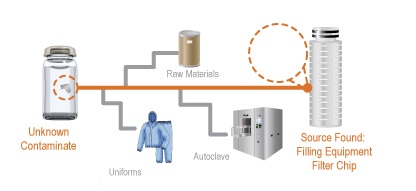
Webinar Recap and Q&A for Determining the Source of Contaminants in a Manufacturing Process
[fa icon="calendar'] Nov 29, 2012 12:50:24 PM / by Admin posted in Blog, foreign particulate matter, Pharmaceutical, pharmaceutical forensics, quality control, source determination
On October 30th, David Exline, Senior V.P. and Antonio Scatena, Laboratory Manager at Gateway Analytical hosted a webinar designed for laboratory and manufacturing personnel that encounter contaminants and foreign particulate matter in the manufacturing, process/product development and customer returns. This webinar discussed the process by which contaminants and foreign particles are isolated, identified, tracked to find the source of the issue, and then addressed to ensure that the issue does not arise again.