Presented at: 2014 IACP Conference
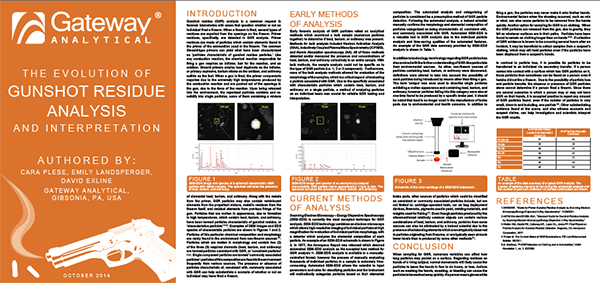
Authors: Cara Plese, Emily Landsperger, David Exline
Release Date: October 2014
The Evolution of Gunshot Residue Analysis
[fa icon="calendar'] Nov 19, 2014 1:10:46 AM / by Cara Plese posted in 2014 IACP poster, Blog, forensic analysis, gunshot residue analysis, Posters & Presentations, SEM/EDS
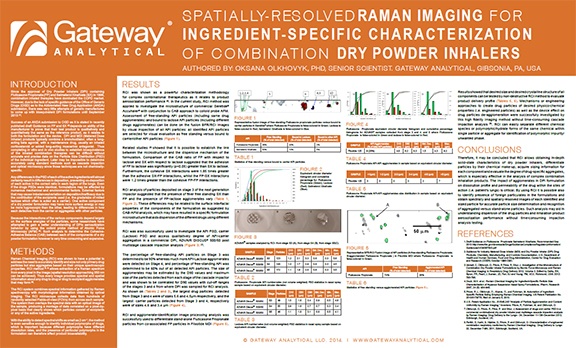
Spatially-Resolved Raman Imaging for Ingredient-Specific Characterization of Combination Dry Powder Inhalers
[fa icon="calendar'] Jun 17, 2014 6:49:12 AM / by Admin posted in aggregates, Blog, DPI formulations, Dry Powder Inhalers, Posters & Presentations
Presented at: 2014 ASSA International IWPCPS-16 Workshops
Author: Oksana Olkhovyk
Release Date: June 16, 2014
Gateway Analytical to Present on Spatially-resolved Raman Imaging for Ingredient-Specific Characterization of Combination Dry Powder Inhalers
[fa icon="calendar'] Jun 12, 2014 1:25:51 PM / by Admin posted in bioavailability, Blog, characterizing DPIs, News Releases, particle characterization, Raman chemical imaging
PITTSBURGH, June 12th, 2014 — Gateway Analytical announced today that they will be presenting a poster session that focused on using Raman Imaging for chemically specific characterization of Combination Dry Powder Inhalers at the Sixteenth International Workshop on Physical Characterization of Pharmaceutical Solids (IWPCPS®-16), June 16-19th in Prague, Czech Republic.
The Written Word: An Important Part of Quality Communication
[fa icon="calendar'] Jun 4, 2014 1:33:16 PM / by Tricia Wood posted in Blog, quality assurance, quality communication, quality control, quality management system
While communication is not the sole responsibility of the quality department, it certainly plays a large role in the management and execution of an effective quality system. Even for the most organized and well documented quality system, successful communication is a must. Although I have discussed effective communication in the past, I want to focus on written communication. In the day and age of texting and social media, it seems as though with all of means for personal interface that we would become better at communication. However, I have noticed when it comes to thorough and precise written communication, it now seems to be a coveted skill rather than an inherent capability.
Gateway Analytical to Promote Particulate Identification Services at the 3rd Annual ISPE-FDA CGMP Conference
[fa icon="calendar'] Jun 2, 2014 5:14:46 PM / by Admin posted in Blog, News Releases
PITTSBURGH, June 2nd, 2014 — Gateway Analytical announced today that they will be exhibiting at the 3rd Annual ISPE-FDA CGMP Conference in Baltimore, Maryland, June 2-4th. The ISPE-FDA CGMP Conference promotes and presents the latest topics in different areas of the pharmaceutical industry including quality, innovation, technology and regulatory affairs. At this event, attendees and exhibitors benefit from having direct interaction and discussions with the FDA and leading experts in the areas of drug development and manufacturing.
Evaluation of Pharmaceutical Vials for Glass Delamination
[fa icon="calendar'] May 16, 2014 3:17:17 PM / by Admin posted in automated particle identification, Blog, Case Studies, glass delamination
Overview
Glass delamination if not discovered early can be big problem for pharmaceutical manufacturers. It has the potential to cause costly product recalls and investigations. Because of this, the FDA highly recommends that manufacturers perform stability studies to evaluate the interaction of the drug formulation with the glass vial in addition to pretesting analysis on smaller batches of the product before going into full production.
Chemically Specific Particle Sizing & Agglomerate Analysis Services
[fa icon="calendar'] May 15, 2014 1:56:56 PM / by Admin posted in aqueous suspension formulations, bioequivalence, Blog, chemically specific analysis, chemically specific particle sizing, Ingredient-Specific Particle Sizing, particle size distribution, particle sizing, pharmaceutical formulation, Raman chemical imaging, Videos
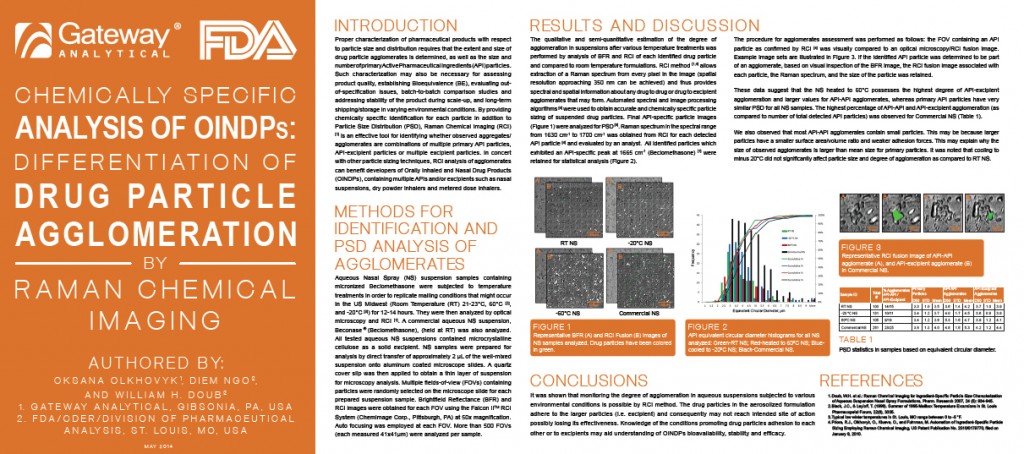
Chemically Specific Analysis of OINDPs: Differentiation of Drug Particle Agglomeration by Raman Chemical Imaging
[fa icon="calendar'] May 8, 2014 6:00:45 AM / by Oksana Olkhovyk posted in Blog, Posters & Presentations
Presented at: Respiratory Drug Delivery (RDD) 2014
Authors: O Olkhovyk1, Diem Ngo 2 and William Doub 2
1. Gateway Analytical, 5316 William Flynn Highway, Gibsonia, PA 15044
2. FDA/CDER/Division of Pharmaceutical Analysis, St. Louis , MO
Release Date: Monday, May 5, 2014
Gateway Analytical to Present with the FDA on Chemically Specific Analysis of Orally Inhaled Drug Products at the Respiratory Drug Delivery Conference in Puerto Rico
[fa icon="calendar'] Apr 24, 2014 6:00:13 AM / by Admin posted in Blog, chemically specific analysis, Drug Particle Agglomeration, FDA submission, Ingredient-Specific Particle Sizing, News Releases, OINDPs, Raman chemical imaging, RDD 2014
PITTSBURGH, April 24th, 2014 — Gateway Analytical announced today that they will be exhibiting and presenting at the upcoming Respiratory Drug Delivery (RDD) conference in Fajardo, Puerto Rico, May 4-8. RDD is an international conference that features in-depth presentations and discussions focused on the latest development in respiratory drug delivery science, in addition to industry networking. During the conference Gateway will be promoting their unique Raman chemical imaging services that provide drug developers with truly chemically specific particle characterization. This chemical specificity is the key to understanding the extend of agglomerations in orally inhaled drug products (OINDPs), which is critical for safety, stability and FDA submission.
Gateway Analytical Collaborates with the FDA to Present their Latest Findings on Temperature-mediated Drug Agglomerates
[fa icon="calendar'] Mar 11, 2014 10:05:55 AM / by Admin posted in Blog, News Releases
PITTSBURGH, March, 11th, 2014 — Gateway Analytical announced today that they have collaborated with researchers from the Division of Pharmaceutical Analysis at the Food and Drug Administration’s Drug Evaluation and Research Center (FDA/CDER) to present new findings on temperature-mediated agglomeration assessment in Orally Inhaled and Nasal Drug Products (OINDP’s) at the upcoming Respiratory Drug Delivery (RDD) conference in Fajardo, Puerto Rico, May 4-8, 2014.