Conference: IFPAC - 2013 27th International Forum and Exhibition Process Analytical Technology
Location: Baltimore, Maryland, U.S.A.
Date: Wednesday, January 23rd during the PM VIII Session
Time: 1 – 5 pm
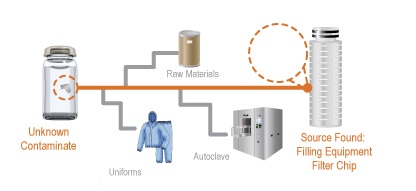
Forensic Analysis of Foreign Particles in Pharmaceutical Materials at the IFPAC Annual Meeting
[fa icon="calendar'] Jan 23, 2013 10:30:09 AM / by Rebekah Byrne posted in Blog, contamination investigations, deviation investigations, foreign particulate matter, non-conformance, Particle Identification in Pharmaceuticals, Pharmaceutical, pharmaceutical forensics
Working to Address the FDA Critical Path Opportunity for Generic Nasal Spray Suspensions
[fa icon="calendar'] Jan 22, 2013 3:27:15 PM / by Admin posted in bioequivalence, Blog, cGMP laboratory, Critical Path Opportunity, Generic nasal spray suspsension, Ingredient-Specific Particle Sizsing, Pharmaceutical, Pharmaceutical Webinars, Raman chemical imaging, Recorded Webinars
Management Review — More Than Just Another Boring Quality Meeting
[fa icon="calendar'] Jan 18, 2013 11:00:11 AM / by Tricia Wood posted in Blog, Quality Assurance, quality assurance, quality control, Quality Corner, quality management, quality management system
By definition, management review is a formal meeting of top management with the purpose of reviewing and evaluating the effectiveness of the quality management system. Yes, I know, it sounds like another humdrum status report meeting. And unfortunately, that is the way it is viewed and conducted by many organizations. Management review is too often regarded as something to check off the list in order to satisfy a standard or an auditor. I must admit, when I first started holding management reviews, I shared that view. I would diligently set my agenda to include all of the required items; then I would simply steamroll through them. I’d write my report, file it away, and then proudly present it to the next auditor to examine. As a result, nothing was really gained and much valuable time was lost.
Gateway Analytical Interview on Pittsburgh Technology Council’s TechVibe Radio
[fa icon="calendar'] Jan 8, 2013 11:04:11 AM / by Admin posted in analytical testing, Blog, forensic expert, forensics, Pharmaceutical, pharmaceutical, pharmaceutical forensics
Recently on December 22nd, Dave Exline, Senior V.P. at Gateway Analytical stopped by the Pittsburgh Technology Council’s TechVibe Radio to talk on-air with Audrey Russo and Jonathan Kersting about Gateway Analytical and its services.
Be Proactive, Not Reactive When it Comes to Your Quality System
[fa icon="calendar'] Dec 18, 2012 9:30:56 AM / by Tricia Wood posted in Blog, Quality Assurance, Quality Corner
One Monday morning in November, an FDA Inspector visited Gateway to perform an inspection. Although we are FDA registered, it came as a complete surprise. It is times like these when you realize how important it is to have a quality management system that is designed to be ‘audit-ready’ at all times. Implementing checks and double checks of all systems (e.g. training, calibration, CAPA, document control, etc.) is crucial for ensuring everything is kept up-to-date per internal procedures and regulatory requirements.
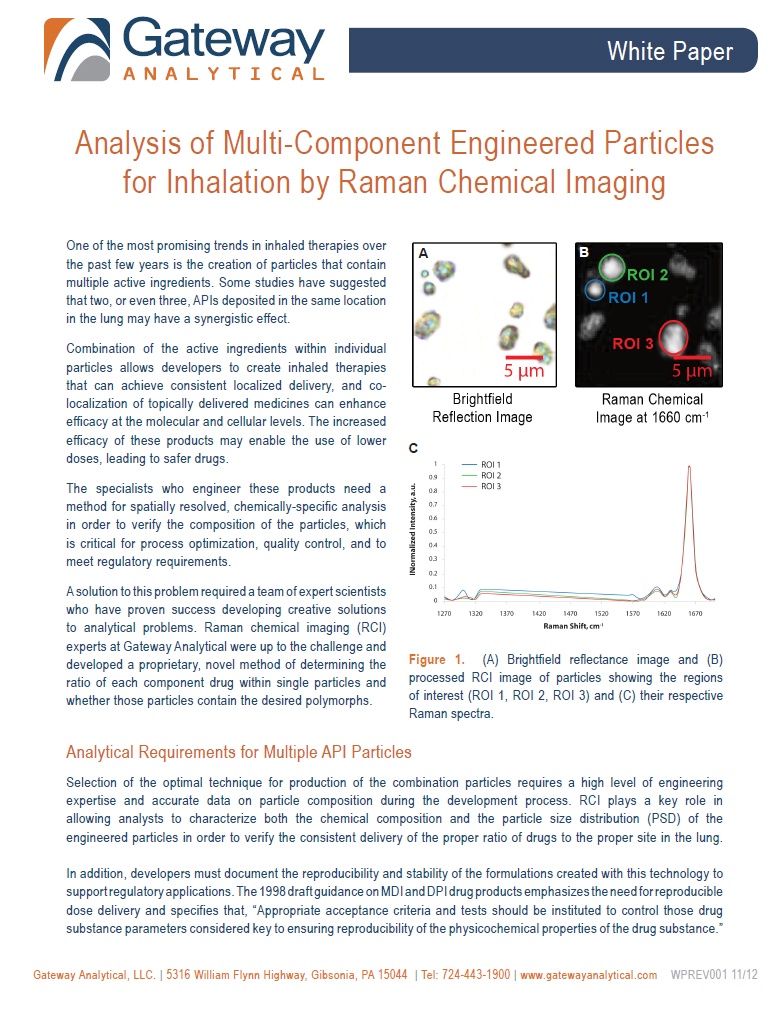
Using Raman Chemical Imaging to Analyze Inhaled Combination Therapies
[fa icon="calendar'] Dec 13, 2012 2:00:54 PM / by Oksana Olkhovyk posted in Articles & Publications, Blog, Pharmaceutical, White Papers
White Paper Preview:
Analysis of Multi-Component Engineered Particles for Inhalation by Raman Chemical Imaging
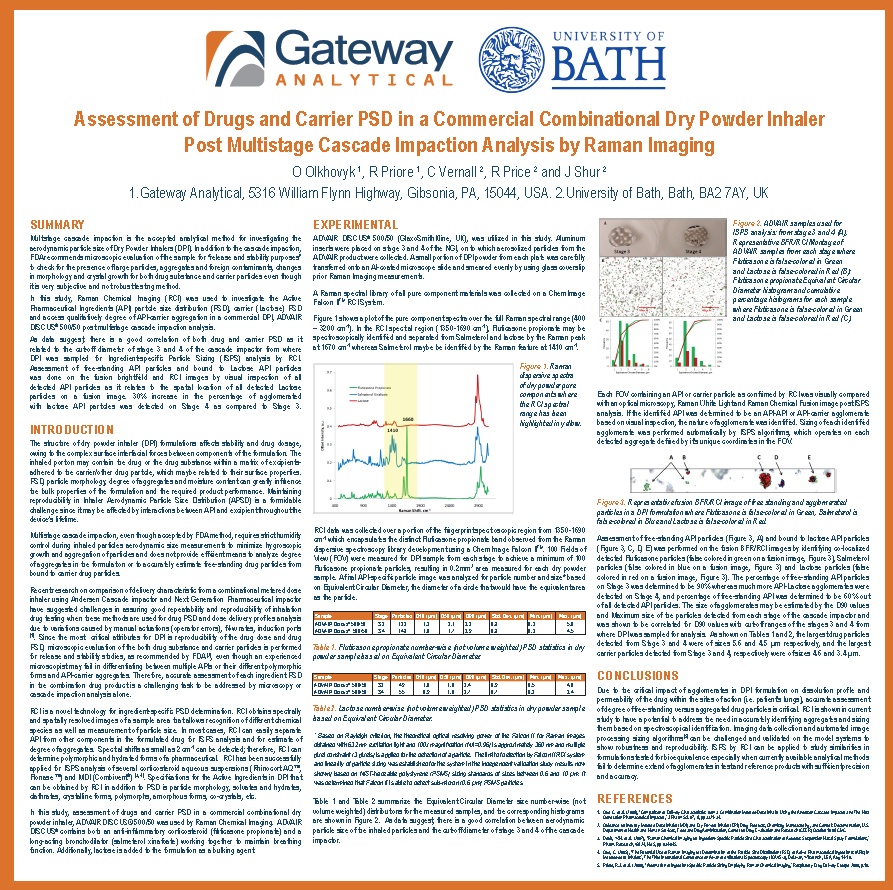
[fa icon="calendar'] Dec 11, 2012 10:30:57 AM / by Oksana Olkhovyk posted in Articles & Publications, Blog, Pharmaceutical, White Papers
White Paper Preview:
Assessment of Drugs and Carrier PSD in a Commercial Combinational Dry Powder Inhaler Post Multistage Cascade Impaction Analysis by Raman Imaging
[fa icon="calendar'] Dec 7, 2012 12:17:16 PM / by Oksana Olkhovyk posted in Blog, Pharmaceutical, Posters & Presentations
Presented at: Drug Delivery to the Lung 23
Authors: O Olkhovyk 1, R Priore 1, C Vernall 2, R Price 2 and J Shur 2
1.Gateway Analytical, 2.University of Bath
Release Date: Wed., December 5, 2012
Webinar Recap and Q&A for Laboratory Methods for Failure Analysis of Pharmaceutical Products
[fa icon="calendar'] Dec 5, 2012 12:05:39 PM / by Admin posted in Blog, failure analysis, nonconformance, Pharmaceutical, pharmaceutical forensics, problem solving, quality assurance, quality control, quality management system
On November 14th, Dave Exline, Senior V.P. and Rebekah Wagurak, Forensic Scientist at Gateway Analytical hosted a webinar designed for laboratory and manufacturing personnel that are responsible for quality assurance and quality control of processes in the manufacturing of pharmaceuticals. This webinar discussed case studies and walked participants through the process of a failure analysis investigation, common methods used, and interpretation of analytical data to improve overall process development and quality control.
Drug Delivery to the Lung 23 Poster Preview
[fa icon="calendar'] Nov 29, 2012 12:50:58 PM / by Oksana Olkhovyk posted in Blog, Pharmaceutical
Poster Title: Assessment of Drugs and Carrier PSD in a Commercial Combinational Dry Powder Inhaler Post Multistage Cascade Impaction Analysis by Raman Imaging
Date: Wed., December 5, 2012
Time: 3:30 pm - 5:00 pm