Gateway Analytical Senior Scientist Oksana Olkhovyk, Ph.D. recently presented at RDD Europe 2013 in Berlin, Germany. The workshop titled: "Automated Ingredient Specific Particle Sizing" is now available for download.
RDD Europe 2013 Workshop: Automated Ingredient Specific Particle Sizing
[fa icon="calendar'] Jun 12, 2013 9:00:42 AM / by Admin posted in bioequivalence, Blog, cGMP, Ingredient-Specific Particle Sizing, ISPS, particle characterization, particle sizing, Pharmaceutical
In Depth Analysis of Particle Contamination for Inhalable Drug Products
[fa icon="calendar'] Jun 4, 2013 6:05:49 PM / by Admin posted in analytical approach, Blog, dave exline, foreign particulate matter, Inhalable drug products, Medical Device Webinar Recordings, particle contamination, Pharmaceutical, Pharmaceutical QA/QC Investigations Webinars, Recorded Webinars
Original Event Date: Tuesday, June 4, 2013
Duration: 45 minutes
Presenters: David Exline, Senior Vice President at Gateway Analytical
Host: Shawn Wilhelm, Marketing Coordinator at Gateway Analytical
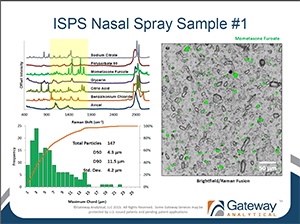
Polymorph Identification of Drug Particles in Orally Inhaled and Nasal Drug Products
[fa icon="calendar'] Jun 4, 2013 10:00:45 AM / by Oksana Olkhovyk posted in Blog, Pharmaceutical, Posters & Presentations
Respiratory Drug Delivery Europe 2013
Authors: Oksana Olkhovyk, Gateway Analytical, 5316 William Flynn Highway, Gibsonia, PA 15044
Release Date: Wed., May 22, 2013
What's in a Word? The Importance of Using the Proper Quality Terminology in the Lab
[fa icon="calendar'] May 31, 2013 10:12:45 AM / by Tricia Wood posted in Blog, Quality Assurance, quality assurance, quality control, Quality Corner, quality management system, quality system
It may seem a little nitpicky to point out the difference between conformance and compliance, validation and qualification, or even registered and certified. After all, how often is someone going to ask you to correctly define compliance and use it properly in a sentence? Unless your employer exercises their right to hand out pop quizzes, probably never.
Recap of the 2013 Mid-Atlantic Association of Forensic Scientists (MAAFS) Conference
[fa icon="calendar'] May 20, 2013 12:02:27 PM / by Rebekah Byrne posted in Blog, Forensics, MAAFS, Mid-Atlantic Association of Forensic Scientists, Rebehak Byrne
Last week, I attended the annual meeting for the Mid-Atlantic Association of Forensic Scientists in Roanoke, Virginia. The meeting was held at the historic Hotel Roanoke and Conference Center, located in the Blue Ridge Mountains. Not only was the drive through the West Virginian mountain country beautiful, but the conference overall was a great experience.
Q&A Recap for In Depth Analysis of Particle Contamination for Parenteral Drug Products
[fa icon="calendar'] May 20, 2013 11:26:01 AM / by Admin posted in Blog, Pharmaceutical, Quality Assurance
On May 14th, Rebekah Byrne, Scientist I and David Exline, Senior Vice President at Gateway Analytical hosted a webinar designed for developers of parenteral drug products. During this webinar, our presenters identified commonly found contaminants in parenteral drug products, how these contaminants are introduced into the manufacturing process, how they can be detected both visibly and non-visibly and laboratory techniques used to identify contaminates. They provided insight into terminology used when discussing parenteral contamination including the difference between particle-free vs. sterile and the standards for visual inspection. They also discussed the importance of taking a pro-active approach to quickly source and manage contaminant issues in the manufacturing process to reduce the risk of future contamination and potentially save your company money by preventing potential recalls.
In Depth Analysis of Particle Contamination for Parenteral Drug Products
[fa icon="calendar'] May 15, 2013 4:40:45 PM / by Admin posted in Blog, manufacturing, Medical Device Webinar Recordings, parenteral drug products, particle contamination, Pharmaceutical, Pharmaceutical QA/QC Investigations Webinars, Recorded Webinars
Original Event Date: Tuesday, May 14, 2013
Duration: 60 minutes
Presenters: David Exline, Senior Vice President & Rebekah Byrne, Scientist I at Gateway Analytical
Host: Shawn Wilhelm, Marketing Coordinator at Gateway Analytical
Value of Presumptive Screening of Physical Evidence Q&A Recap
[fa icon="calendar'] May 7, 2013 11:25:02 AM / by Admin posted in Blog, forensic analysis, Forensics, gunshot residue, microscopic analysis, physical evidence, presumptive screening, trace evidence, Trace Evidence
On April 30th, Dave Exline, Senior V.P. and Cara Plese, M.S., Scientist I at Gateway Analytical hosted a webinar designed for police and attorneys involved in cases with physical evidence. During this webinar, our presenters discussed the advantages of microscopic and presumptive screenings of physical evidence, especially suspected bodily fluid stains and hair prior to advancing to more expensive DNA analysis. They also provide examples of such screenings and discussed how these screening methods can save valuable time, money and resources by eliminating samples from being forwarded onto unnecessary further analysis.
The Value of Presumptive Screening of Physical Evidence
[fa icon="calendar'] May 6, 2013 12:00:32 PM / by Admin posted in Blog, Forensics, Forensic Webinar Recordings, microscopic analysis, physical evidence, presumptive screening, Recorded Webinars
When Do You Bring in a Consultant?
[fa icon="calendar'] Apr 19, 2013 10:35:50 AM / by David Exline posted in Blog, Consultant Services
The consultant is a valuable tool for any organization if properly utilized. The key to gaining the most benefit to using a consultant starts with the need. The need must be clearly identified as a one time need or ongoing need. The organization must first determine if the appropriate expertise is represented within the organization. If the specific expertise is present in house, then one must ask if outside help can add value to existing group. Often times for specific problems, a consultant may be the right approach.