Inhalation and Nasal Technology Focus Group (INTFG) Annual Fall Symposium Event Recap
[fa icon="calendar'] Sep 27, 2012 12:25:36 PM / by Admin posted in AAPS, Blog, INTFG, Nasal Drug Products, Nasal suspension PSD, Pharmaceutical, pharmaceutical, pharmaceutical formulation
Course Review - Manufacturing & Testing of PSA Tapes
[fa icon="calendar'] Sep 20, 2012 12:00:58 PM / by Rebekah Byrne posted in Blog, Forensics, Trace Evidence
Trace evidence encompasses a number of possible sample types, from fibers to hairs to gunshot residue. One of the commonly encountered types of trace evidence is tape evidence. In order to properly understand tape from a trace evidence perspective, it is helpful to understand the manufacture process of the product.
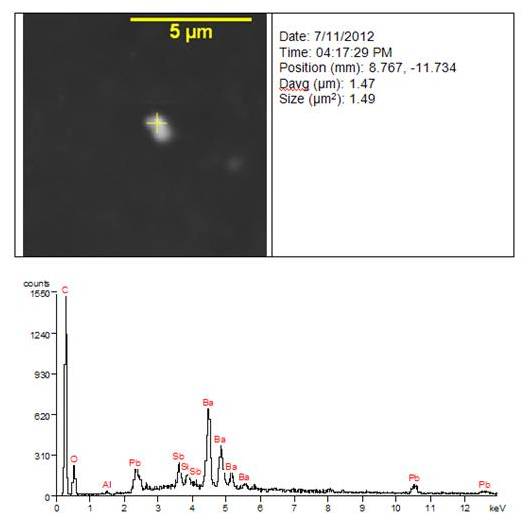
Method Validation Study for the Preparation and Analysis of Ingredient-Specific Particle Sizing by Raman Chemical Imaging as it Relates to the Generic Nasal Spray Suspensions
[fa icon="calendar'] Sep 14, 2012 2:08:04 PM / by Oksana Olkhovyk posted in Blog, Pharmaceutical, Posters & Presentations
https://cdn2.hubspot.net/hubfs/2753637/Webinars,%20Brochures%20and%20Publications/Posters%20and%20Presentations/2016%20and%20Prior%20Years/2012/2012-INTFG-Poster-Gateway-rev3-FINAL.pdfPresented at: The 2012 Inhalation and Nasal Technology Focus Group (INTFG) 18th Annual Fall Symposium
Authors: Oksana Olkhovyk and Ryan Priore – Gateway Analytical, 5316 William Flynn Highway, Gibsonia, PA 15044
Release Date: Sept. 6, 2012
Webinar Recap: Polymorph Analysis Using Raman Chemical Imaging
[fa icon="calendar'] Aug 31, 2012 2:51:19 PM / by Admin posted in Blog, Pharmaceutical
In our latest webinar on Thursday, Aug. 23rd, Gateway Analytical's Senior Scientist Oksana Olkhovyk presented a webinar designed for scientists working in the area of polymorph evaluation in pharmaceutical products and discussed the benefits of using Raman microscopy and chemical imaging for in vitro characterization of polymorphs.
An Update on the ASCLD/LAB-International Accreditation Process
[fa icon="calendar'] Aug 24, 2012 10:30:30 AM / by Tricia Wood posted in ASCLD/LAB-International, Blog, quality assurance, quality control, quality system
Gateway Analytical is currently working through the final stages of the ASCLD/LAB-International accreditation process. After completing the initial stages as well as the on-site audit, compliance with the standards of ASCLD/LAB are continuing to be demonstrated through both the written procedures and practices at the laboratory. Upon final review of all items needed for accreditation approval, Gateway Analytical will be operating in compliance with the guidelines outlined by both ASCLD/LAB-International and ISO 17025:2005. All sub-disciplines, upon final review and approval, will fall under the scope of trace evidence testing.
Webinar Recap: Forensic Analysis of Paint and Tapes for Police & Attorneys
[fa icon="calendar'] Aug 22, 2012 11:00:36 AM / by Admin posted in Blog, crime scene investigation, forensic expert, Forensics, forensics, paint analysis, tape analysis, trace evidence, Trace Evidence
On August 8th, David Exline, Senior V.P. & Court-Qualified Trace Evidence Expert and Rebekah Wagurak, Forensic Scientist at Gateway Analytical presented a webinar that covers the forensic investigation process for paint and tape evidence. Topics include the most current methods used for today’s investigators and discuss the interpretation of paint transfer evidence. Case studies related to the analysis of duct tapes, automotive and architectural paints were also discussed.
Analysis of Particle Agglomeration and Content Uniformity by Raman Imaging
[fa icon="calendar'] Jul 30, 2012 1:59:42 PM / by Oksana Olkhovyk posted in Blog, Pharmaceutical, Posters & Presentations
Presented at: The 39th Annual Meeting & Exposition of the Controlled Release Society (CRS 2012)
Authors: Oksana Olkhovyk and Ryan Priore - Gateway Analytical, 5316 William Flynn Highway, Gibsonia, PA 15044
Release Date: July 16, 2012
Gateway Analytical Receives Controlled Substance Registration Certificate from the Drug Enforcement Administration
[fa icon="calendar'] Jul 26, 2012 4:20:53 PM / by Rebekah Byrne posted in Blog, Controlled Substance Registration, DEA, drug scheduling, Pharmaceutical, pharmaceutical forensics
Gateway Analytical is proud to announce that we have obtained our Controlled Substance Registration Certificate issued by the United States Department of Justice Drug Enforcement Administration’s Office of Diversion Control.
Acquisition of Third SEM-EDS System will be Used Exclusively for the Analysis of Forensic Gunshot Residue Evidence
[fa icon="calendar'] Jul 13, 2012 10:40:21 AM / by Rebekah Byrne posted in Blog, crime scene investigation, energy dispersive spectroscopy, forensic expert, Forensics, forensics, GSR analysis, gunshot residue analysis, Gunshot Residue Evidence, scanning electron microscope, trace evidence, Trace Evidence, sem-eds
Webinar Recap: Ensure Quality Inhalation Products with Wear Debris Analysis of Medical Devices
[fa icon="calendar'] Jun 26, 2012 10:51:37 AM / by Admin posted in analytical testing, Blog, Nasal Drug Products, Nasal suspension PSD, Pharmaceutical, problem solving, quality control, quality management system, wear debris testing
On May 30th, David Exline, Senior V.P. at Gateway Analytical presented a webinar that covered an overview of wear debris characterization for laboratory personnel that evaluate quality issues with medical devices. This webinar focused on the causes of wear debris, the affects of such debris on product quality, acceptable limits and methods for ensuring quality products.