Admin
Recent Posts
What Police and Attorneys Need to Know about Condom Lubricant Analysis
[fa icon="calendar'] Aug 6, 2013 9:40:58 AM / by Admin posted in Blog, condom lubricant analysis, forensic analysis, Forensics, Forensic Webinar Recordings, trace evidence, trace evidence analysis
Now What?: After the Accreditation Celebration
[fa icon="calendar'] Jul 25, 2013 10:00:13 AM / by Admin posted in accreditation, Blog, continual quality improvement, ISO 17025, ISO 9001, Quality Assurance, Quality Corner, quality management system
The day the certificate arrives in the mail, it finally sinks in – all that hard work, extra time in the office, hours spent preparing has finally paid off! As you adjust to the idea that the fruits of your labor have finally (FINALLY!) been recognized, another realization begins to come to light. Along with the satisfaction of hanging your accreditation certificate on the wall, you've also come to the realization that the journey has just begun. As with any complex and detailed structure, your newly accredited quality management system is going to need continued TLC to guarantee its success.
Q&A Recap for Identify Agglomerates by their Chemical Makeup using Raman Chemical Imaging
[fa icon="calendar'] Jul 15, 2013 3:46:32 PM / by Admin posted in Blog, Pharmaceutical
On July 10th, Oksana Olkhovyk, Ph.D., Senior Scientist at Gateway Analytical hosted a webinar designed for scientists working to develop various pharmaceutical drug products. During this webinar, we discussed how Raman Chemical Imaging (RCI) is an effective tool that can be used to identify agglomerates by their chemical makeup in various drug formulations, and how it can benefit developers of OINDPs such as nasal spray suspensions, dry powder inhalers (DPIs), metered dosed inhalers (MDIs), as well as semi-solids: topical creams, emulsions and gels. We also discussed the validation of the RCI method to objectively and precisely evaluate the extent and size of drug to drug or drug to excipient aggregates and compare it to other methods for identifying agglomerates including, optical microscopy, Anderson Cascade Impaction, Raman Microscopy and Confocal Spectroscopy.
Identify Agglomerates by their Chemical Makeup using Raman Chemical Imaging
[fa icon="calendar'] Jun 13, 2013 9:13:44 AM / by Admin posted in Agglomerates, Blog, Dry Powder Inhalers, metered dosed inhalers, nasal spray suspensions, OINDPs, Orally Inhaled and Nasal Drug Products, Pharmaceutical, Pharmaceutical Webinars, Raman chemical imaging, Recorded Webinars
Original Event Date: Wednesday, July 10, 2013
Duration: 60 minutes
Presenters: Oksana Olkhovyk, Ph.D., Senior Scientist at Gateway Analytical
Host: Shawn Wilhelm, Marketing Coordinator at Gateway Analytical
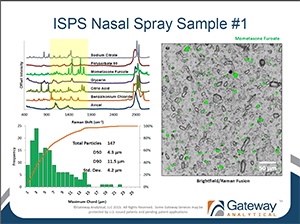
RDD Europe 2013 Workshop: Automated Ingredient Specific Particle Sizing
[fa icon="calendar'] Jun 12, 2013 9:00:42 AM / by Admin posted in bioequivalence, Blog, cGMP, Ingredient-Specific Particle Sizing, ISPS, particle characterization, particle sizing, Pharmaceutical
Gateway Analytical Senior Scientist Oksana Olkhovyk, Ph.D. recently presented at RDD Europe 2013 in Berlin, Germany. The workshop titled: "Automated Ingredient Specific Particle Sizing" is now available for download.
In Depth Analysis of Particle Contamination for Inhalable Drug Products
[fa icon="calendar'] Jun 4, 2013 6:05:49 PM / by Admin posted in analytical approach, Blog, dave exline, foreign particulate matter, Inhalable drug products, Medical Device Webinar Recordings, particle contamination, Pharmaceutical, Pharmaceutical QA/QC Investigations Webinars, Recorded Webinars
Original Event Date: Tuesday, June 4, 2013
Duration: 45 minutes
Presenters: David Exline, Senior Vice President at Gateway Analytical
Host: Shawn Wilhelm, Marketing Coordinator at Gateway Analytical
Q&A Recap for In Depth Analysis of Particle Contamination for Parenteral Drug Products
[fa icon="calendar'] May 20, 2013 11:26:01 AM / by Admin posted in Blog, Pharmaceutical, Quality Assurance
On May 14th, Rebekah Byrne, Scientist I and David Exline, Senior Vice President at Gateway Analytical hosted a webinar designed for developers of parenteral drug products. During this webinar, our presenters identified commonly found contaminants in parenteral drug products, how these contaminants are introduced into the manufacturing process, how they can be detected both visibly and non-visibly and laboratory techniques used to identify contaminates. They provided insight into terminology used when discussing parenteral contamination including the difference between particle-free vs. sterile and the standards for visual inspection. They also discussed the importance of taking a pro-active approach to quickly source and manage contaminant issues in the manufacturing process to reduce the risk of future contamination and potentially save your company money by preventing potential recalls.
In Depth Analysis of Particle Contamination for Parenteral Drug Products
[fa icon="calendar'] May 15, 2013 4:40:45 PM / by Admin posted in Blog, manufacturing, Medical Device Webinar Recordings, parenteral drug products, particle contamination, Pharmaceutical, Pharmaceutical QA/QC Investigations Webinars, Recorded Webinars
Original Event Date: Tuesday, May 14, 2013
Duration: 60 minutes
Presenters: David Exline, Senior Vice President & Rebekah Byrne, Scientist I at Gateway Analytical
Host: Shawn Wilhelm, Marketing Coordinator at Gateway Analytical
Value of Presumptive Screening of Physical Evidence Q&A Recap
[fa icon="calendar'] May 7, 2013 11:25:02 AM / by Admin posted in Blog, forensic analysis, Forensics, gunshot residue, microscopic analysis, physical evidence, presumptive screening, trace evidence, Trace Evidence
On April 30th, Dave Exline, Senior V.P. and Cara Plese, M.S., Scientist I at Gateway Analytical hosted a webinar designed for police and attorneys involved in cases with physical evidence. During this webinar, our presenters discussed the advantages of microscopic and presumptive screenings of physical evidence, especially suspected bodily fluid stains and hair prior to advancing to more expensive DNA analysis. They also provide examples of such screenings and discussed how these screening methods can save valuable time, money and resources by eliminating samples from being forwarded onto unnecessary further analysis.
The Value of Presumptive Screening of Physical Evidence
[fa icon="calendar'] May 6, 2013 12:00:32 PM / by Admin posted in Blog, Forensics, Forensic Webinar Recordings, microscopic analysis, physical evidence, presumptive screening, Recorded Webinars