On November 14th, Dave Exline, Senior V.P. and Rebekah Wagurak, Forensic Scientist at Gateway Analytical hosted a webinar designed for laboratory and manufacturing personnel that are responsible for quality assurance and quality control of processes in the manufacturing of pharmaceuticals. This webinar discussed case studies and walked participants through the process of a failure analysis investigation, common methods used, and interpretation of analytical data to improve overall process development and quality control.
Webinar Recap and Q&A for Laboratory Methods for Failure Analysis of Pharmaceutical Products
[fa icon="calendar'] Dec 5, 2012 12:05:39 PM / by Admin posted in Blog, failure analysis, nonconformance, Pharmaceutical, pharmaceutical forensics, problem solving, quality assurance, quality control, quality management system
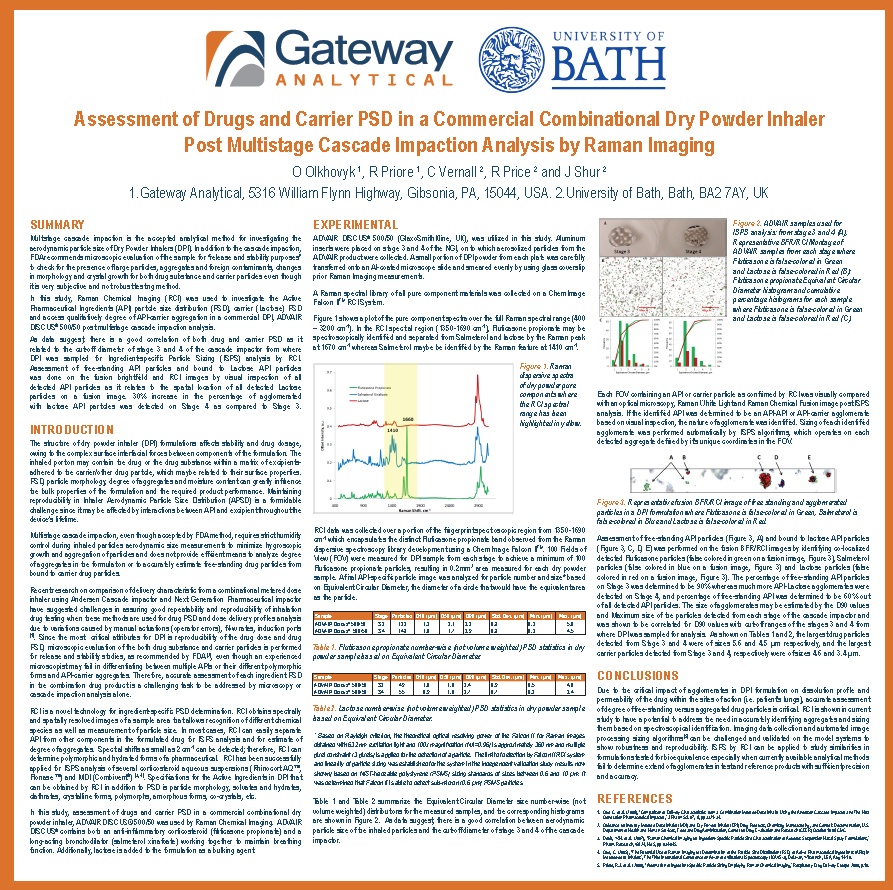
Drug Delivery to the Lung 23 Poster Preview
[fa icon="calendar'] Nov 29, 2012 12:50:58 PM / by Oksana Olkhovyk posted in Blog, Pharmaceutical
Poster Title: Assessment of Drugs and Carrier PSD in a Commercial Combinational Dry Powder Inhaler Post Multistage Cascade Impaction Analysis by Raman Imaging
Date: Wed., December 5, 2012
Time: 3:30 pm - 5:00 pm
Webinar Recap and Q&A for Determining the Source of Contaminants in a Manufacturing Process
[fa icon="calendar'] Nov 29, 2012 12:50:24 PM / by Admin posted in Blog, foreign particulate matter, Pharmaceutical, pharmaceutical forensics, quality control, source determination
On October 30th, David Exline, Senior V.P. and Antonio Scatena, Laboratory Manager at Gateway Analytical hosted a webinar designed for laboratory and manufacturing personnel that encounter contaminants and foreign particulate matter in the manufacturing, process/product development and customer returns. This webinar discussed the process by which contaminants and foreign particles are isolated, identified, tracked to find the source of the issue, and then addressed to ensure that the issue does not arise again.
Gateway Analytical Achieves the Prestigious ASCLD/LAB-International Accreditation for Forensic Trace Evidence Testing!
[fa icon="calendar'] Nov 27, 2012 2:12:37 PM / by Tricia Wood posted in ASCLD/LAB accreditation, ASCLD/LAB-International accreditation, Blog, forensic expert, Forensics, forensics, GSR analysis, gunshot residue analysis, ISO 17025, quality assurance, quality control, trace evidence, Trace Evidence
We are now one (1) of only two (2) private labs in the country that is accredited to this standard for trace evidence, and we are the only private lab in the country to be accredited for gunshot residue analysis.
What Lawyers and Judges Need to Known about the Science behind Trace Evidence Examination
[fa icon="calendar'] Nov 15, 2012 11:00:30 AM / by Admin posted in Blog, Evidence Transfer, forensic investigations, forensic science, Forensics, Scientific Method, trace evidence, Trace Evidence Examination, Trace Evidence, Upcoming Events
At the upcoming AAFS Annual Meeting, which is being held on February 18-23, 2013 in Washington, DC, Gateway Analytical will be giving a presentation on "What Lawyers and Judges Need to Known about the Science behind Trace Evidence Examination". Authored by Cara Plese, Antonio Scatena, and David Exline this presentation will help attendees gain a general introduction and exposure to the scientific method employed in the analysis of trace evidence, as well as the conclusions that can be expected from the various types of trace evidence examinations.
"Dine in the Sky" VIP Event in Chicago Recap
[fa icon="calendar'] Nov 12, 2012 10:30:52 AM / by Admin posted in Blog, Community, Pharmaceutical
Webinar Recap & Q&A for Content Uniformity Using Raman Chemical Imaging
[fa icon="calendar'] Oct 30, 2012 10:30:38 AM / by Admin posted in Blog, Pharmaceutical
On September 26, Oksana Olkhovyk, Ph.D., Senior Scientist at Gateway Analytical presented a webinar focused for scientists working to develop oral drug tablets, semi-solid creams and gels, and medical devices such as transdermal patches. In this webinar, she discussed the benefit of Raman chemical imaging (RCI) when evaluating content and blend uniformity. During the webinar, specific question were asked from the audience. You can find the transcribed Q&A below.
Essential Good Analytical Practice with Laboratory Equipment Qualification
[fa icon="calendar'] Oct 24, 2012 10:30:38 AM / by Tricia Wood posted in analytical instrument qualification, Blog, Design Qualification, Installation Qualification, Laboratory Equipment Qualification, Operational Qualification, Performance Qualification, Quality Assurance, quality assurance, quality control, Quality Corner, quality management system, quality system
Laboratory equipment qualification, also referred to as analytical instrument qualification (AIQ), is an essential component of good analytical practice. It provides assurance that the instrument will perform accurately and consistently in accordance with specifications and user requirements. The ultimate objective for equipment qualification is the documented and demonstrated assurance that the unit will produce valid data. Qualifications must be performed initially and on an ongoing basis (no less than annually).
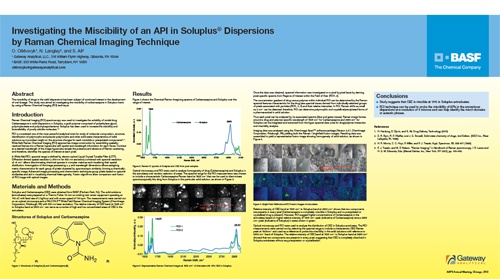
Investigating the Miscibility of an API in Soluplus Dispersions by Raman Chemical Imaging Technique
[fa icon="calendar'] Oct 18, 2012 10:30:21 AM / by Oksana Olkhovyk posted in Blog, Pharmaceutical, Posters & Presentations
Presented at: 2012 AAPS Annual Meeting and Exposition
Authors: Oksana Olkhovyk1, Nigel Langley2, Shaukat Ali2, 1. Gateway Analytical, 2. BASF
Release Date: October 16, 2012
AAPS Poster Session: Investigating the Miscibility of an API in Soluplus Dispersions by RCI Technique
[fa icon="calendar'] Oct 11, 2012 2:32:21 PM / by Admin posted in Blog, Miscibility of Drugs, oral dosage, Pharmaceutical, Raman chemical imaging, solid dispersions
Poster Session: Investigating the Miscibility of an API in Soluplus® Dispersions by Raman Chemical Imaging Technique
Date: Tuesday, October 16, 2012
Time: 9:30 am - 12:30 pm
Location: Hall F